Apple Books
Kindle Books

1 Bonding and Hybridization
When, every student should understand:
- the subject is vast but logical because of unifying facts and concepts
- facts and concepts at the beginning come up repeatedly
- different learning approaches are required for memorizing facts, understanding concepts, and applying both to problems
- facts are important but a small part of the subject
- concepts are critical, but a small part too
- organic chemistry is mostly practicing recalling facts and applying concepts to solve problems
2 Saturated Acyclic Hydrocarbons
Students learn what they think about. They see and read more, but only a fraction is learned. This series of workbooks, when used properly, forces students think about answers, by working problems: lots of them! My answers are online for immediate feedback. Videos are inset in the book to help with concepts.
This particular book is about how and why we draw the longest linear carbon chain as a zigzag, not using the symbols “C” or “H”. This is a fundamental skill for the whole of organic chemistry: chemistry students must know this!

3 Fragments and Functional Groups
This eBook helps with three fundamental concepts for organic chemistry: (i) being able to draw functional groups showing the bonds when none are given: (ii) names of functional groups; and, abbreviations for fragments
It is important to learn names of functional groups and fragments early in organic chemistry, otherwise when a lecturer or text refers to them, students have to look them up to understand, or carry on not understanding. It is a huge advantage to learn them early because they come up repetitively: students who learn them early get ahead on the curve, others fall behind, but all who persevere with the course learn a lot of them. It is not difficult. Just as when dentists tells patients to expect “minor discomfort”; they mean “it is going to hurt a bit, but not as much as if you did not get this fixed”.

4 Conformations of Cyclic Hydrocarbons
Cyclic hydrocarbons have shapes that vary with ring size; some are in equilibrium between two different shapes. This eBook in the series is to help students test if they understand cyclohexanes, cyclopentanes, cyclobutanes, and cyclopropanes.
5 Curly Arrows Showing Electron Flow
Chemical reactivity is the movement of electrons between molecules or molecular fragments. We depict this in organic chemistry using curly arrows. This book is to help students learn how to do this, using lots of problems with online answers.

6 Resonance
Electrons in conjugated anions and cations are not stationary: they flow like waves backwards and forwards. Like a plucked guitar string, these waves have higher amplitudes on some atoms than others. We use resonance structures to predict which atoms bear most of negative or positive charge, and this eBook is to help students learn how. It introduces the impacts of resonance on basicity, situations in which it is unfavorable and favorable in neutral molecules, and an illustrative example of resonance in biochemistry.
7 Acids and Bases
Many transformations in organic chemistry begin by protonating (or adding a Lewis acid to) substrates if the reaction is done under acidic conditions. If basic conditions are used the first step is often deprotonation of something to make it more nucleophilic. This workbook is to introduce interactions of acids and bases with organic compounds.

8 Priority Rules
Assignments of priorities enable understanding of regio- and stereo-isomers. Students struggle with all other aspects of stereochemistry if they do not understand this, and cannot properly understand enantiomers, diastereomers, and epimers if they do not. Priority rules are applied to many organic molecules and the vast majority of biomolecules (many of which should be taught in introductory organic chemistry like carbohydrates, nucleic acids, amino acids, peptides, proteins, and steroids. Learning and teaching priority rules properly at the beginning ot introductory organic chemistry is essential.

9 Chiral Molecules
Molecules with just one chiral center comprise enantiomers. Equal amounts of enantiomers are racemic, if one predominates over the other then there is optical activity due to the enantiomeric excess (ee) of one over the other. One pure enantiomer is optically pure (100% ee). The definition of ee seems over complicated. For instance, why describe a 95:5 mixture of enantiomers as having 90% ee). However, defining things that way makes perfect sense when reading optical purities on a polarimeter because rotation of plane polarized light due to the minor enantiomer (5% in this case) cancels 5% of the rotation due to the major one, so 90% of the maximum value is read.

10 SN1 Displacements At sp3 Centers
SN1 reactions are one of about five mechanisms students are expected to know to pass the first part of sophomore organic chemistry. This ebook covers that process via guided questions. Students who can answer these questions should have no trouble answering any on the exam regarding SN1 reactions.

11 SN2 Nucleophilic Displacement
Learning five key mechanisms is key to passing organic chemistry 1, and SN2 displacements is one of these. SN2 is not one thing, it is a concept. Students must be totally fluent in aspects of it. How to use arrows to describe the mechanism. The kinetics, stereochemistry, how to form SN2 products, and some of the reactions those products can be used for. This ebook covers all of that via a series of guiding questions. The answers are online at by inquisition.org. Students who can answer these questions should have no trouble answering any on the exam regarding SN2 reactions. Enjoy!

12 Eliminations
E1 and E2 eliminations parallel SN1 and SN1, and all four of these processes can compete with each other. Passing the first semester of organic chemistry usually requires not just learning these mechanisms, but recognizing how they can occur under different conditions and with different substrates. This is mastery of concepts. This work book series is designed to help students master concepts by encountering varied examples in self-study problems. Students who work the problems in this book without looking at the answers (www.byinquisition.org) will have no problem answering similar ones on an exam or final.
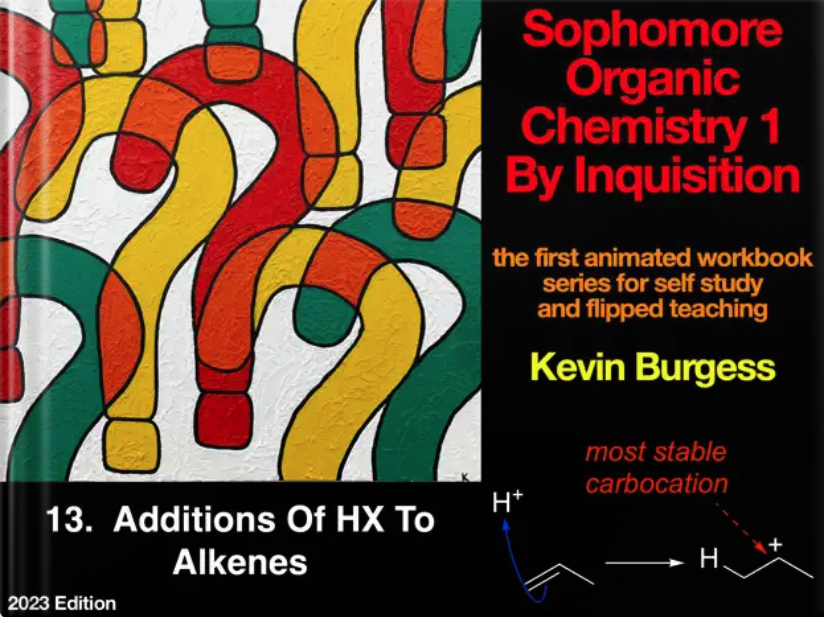
13 Additions of HX to Alkenes
Proton is the simplest electrophile, so let’s use it to understand attack of an electrophile (eg a proton) on a nucleophile (eg an alkene). There is more to this than it appears. First, the reaction is most facile when the carbocation formed is relatively stable, but what governs that? To answer it helps to introduce molecular orbital (MO) theory, and that leads to similar MO factors that govern alkene stabilities. Alkene stabilities are measured by heats of hydrogenation, and that is discussed too. Reactions of protons with alkenes causes some of them to isomerize, to more stable alkenes via more stable cations. Those pesky carbocations can also rearrange via alkyl shifts, a move that can take us by surprise and makes products more difficult to explain if it does. But let’s keep it simple to finish: acid mediated hydration of alkenes to water.

14 Oxidation States
Carbon tetrachloride and carbon dioxide are formally in the same oxidation state, as are methanal and dichloromethane. Comparisons like this, between series, confuses many students at first. This book clarifies this, then looks at ways hydrogen is added to molecules: hydrogenations, hydrogenolyses, and stepwise addition of hydride then protons. Recognizing these processes is more valuable than learning catalysts and conditions. Consequently, concepts are the focus here; are no catalysts or conditions to remember. Numbers of molecules of dihydrogens added relates to the surprisingly useful concept of double bond equivalents (DBEs). DBEs and oxidation states are similar DBEs but different concepts, and this book shows how. Oxidation states are useful when predicting products because molecules only move up and down when oxidizing or reducing agents are added. Conversely, DBEs are helpful for deducing structures when formulae are known (eg from mass spectrometry). Both these concepts are widely useful, it helps to understand them, and this book is designed to help. It does this via leading questions (answers to all online at www.byinquisition.org) and several embedded videos to elaborate key points.

15 Oxidation States
Questions about halogenation of alkenes nearly always come up in sophomore organic chemistry. Chemistries of alkene chlorinations and brominations involve halonium intermediates. Iodinations too, but the products are unstable relative to the starting material; nevertheless, iodination is impactful for alkene isomerization.
When stereospecifities occur in chlorinations and brominations they are governed by halonium ion intermediates. It is easy to see effects of this for cyclic alkenes which give trans-products, but for acyclic alkenes it is necessary to understand how E- and Z-isomers form diasteromeric products via halonium ion intermediates.
Halonium ions can be intercepted in nucleophilic solvents to give halohydrins. Regiochemistries for unsymmetrical alkenes are determined by relative stabilities of transitions states leading to formation of halohydrins from halonium ions.

16 Epoxides And Regioselectivity
Different reaction mechanisms may apply under basic (or basic) and acidic conditions. This is important for epoxides and absolutely vital for carbonyl chemistry (featured later in this series). Under neutral and basic conditions nucleophiles tend to add directly to electrophilic substrates. However, under acidic conditions nucleophiles can be deactivated by protonation, but oxygen atoms on the substrates (here epoxides, later carbonyls) tend to be activated because they are also protonated.

17 Cycloadditions
In a special set of organic reactions, one molecule adds to another to form a ring without any apparent reason. Polar reactions are driven by electrostatic affinity of positive and negative charges, radical reactions by energy stabilizations from pairing unpaired spins, but many cycloadditions are mysteriously different. Some take place in a single step, ie they are concerted, in processes controlled by interactions of highest occupied molecular orbitals (HOMOs) and lowest unoccupied molecular orbitals (LUMOs); these are the “frontierorbitals” and such reactions are “orbital controlled”. Classically this area is illustrated by Diels Alder reactions, but more recently cycloadditions of organic azides to alkynes have become emerged as important to many branches of biomedical science. Understanding these processes earned several people Nobel Prizes (1966 and 2022). Now it is important for people studying introductory organic chemistry to understand these types of reactions, and this workbook is designed to help.