A. The Problem
Why do many students find Sophomore Organic Chemistry difficult?
I teach sophomore organic chemistry frequently, but no one ever taught me how to teach, and few students are taught how to learn any subject, including chemistry. To improve my approach, I started studying the psychology of learning, teaching, and education in general.
Many psychology experiments are relevant, and I had already arrived at some of their conclusions by trial and error. However, some findings were new to me. Somehow, what psychology tells us cannot be the whole story for chemistry though, there must be more to specific to that subject.
Unfortunately, few psychology studies focus on organic chemistry. Most are centered on experimental design more than on the material. Is there something exceptional about sophomore organic chemistry content which makes it particularly hard?
Some students claim organic chemistry is just a lot of memory work. However, only very poorly structured syllabi for organic chemistry stress memorization above all else; there is more to it.
What if the problem was not “memory work” but instead it is the subject that requires memorization tactics and some for different learning approaches too. If that is the case, then identifying types of material, before learning or understanding them becomes critical.
There are at least three distinct types of material in sophomore organic chemistry, and the best strategies to use to learn them are different. Superior ways to teach and learn, in fact, vary with the type of material.
Strategies to learn organic chemistry change according to the type of material within it that is currently being studied. The order they should be studied is also vital.
Memory work and concept understanding are (and should be) stressed in the initial stages of the course; organic chemistry is bewildering if they are not grasped until students have mastered some key facts and conceptual frameworks. Shortly after that, however, the emphasis of sophomore organic shifts towards problem-solving using those concepts and facts gained early on. Students cannot be expected to know this, they have not done the course before, but don’t worry, I am here, and you are reading this.
B. Memory Work: The Foundation Material Type
Some sophomore ORGO is memorization. For instance, early in the course, an instructor might present a bewildering number of functional groups in a table. Lectures delivered in a conventional way (and most do), ie presents for a student to copy, this creates a dilemma.

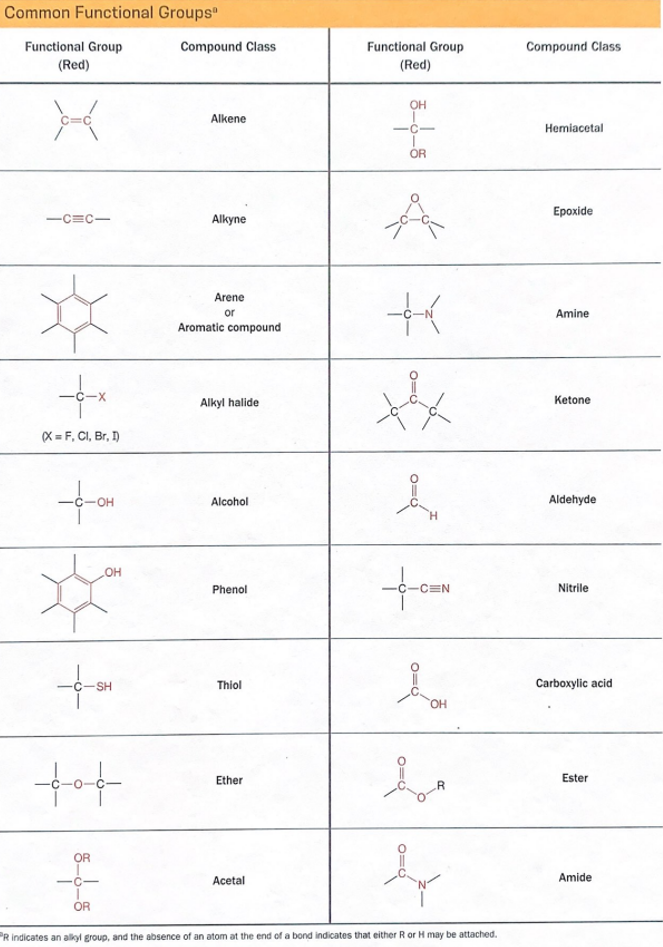
Students look at the table, and think, “No way, you cannot expect us to learn this.” Good lecturers know students are thinking this and must agree, students cannot learn this unless they go home and study it, often and frequently. Memorizing those functional groups is difficult and boring for people who do not know much about them yet. What happens then is the lecturer cannot force the knowledge into the student’s heads, and usually gives up and simply moves on without trying.
In reality, though, students should learn the names and structures of the functional groups early in the class. They will probably know them by the end, but there is so much misery in between if a student has to, for instance, draw on their knowledge of the spectroscopy of a ketone, when they cannot remember what a ketone is. This is at least one reason why sophomore organic is hard, but just a fraction of the difficulty in the big picture.
There are 18 functional groups in that table, and psychology says most people can only remember about three facts in a series, a few more, and they switch off (less than three for someone with attention deficit disorder, a few more for some people). It takes time and effort to memorize and retain material, so what is the best way? Flashcards.
Applications like ANKI present flashcards in orders that allow students to test themselves at frequent intervals (spaced repetition). Decks of flashcards representing mixtures of ORGO topics interleave the materials. Spaced recognition and interleaving are key strategies psychological strategies for memory work.
Unfortunately, ANKI appreciation has not reached organic chemistry to the extent it should, even though medical and law students (who memorize vast arrays of facts) are all over them.
Organic chemistry topics which require memorization include:
• functional groups
• shapes of sp, sp 2 , and sp 3 hybrids
• mathematical definition of pH
Consider memorization of this:
went beautiful the pea pussy in green owl sea cat in
That’s difficult and boring. However, what about this? …..
The owl and the pussy cat went to sea in a beautiful pea-green boat.
The second phrase puts the words that otherwise seem random into a context they understand.
This is the idea behind “memory palaces”: the poorly kept secret that enables some people to do crazy things, like remember hundreds of random words read to them once over a few minutes. Those people are not geniuses (but they are not dumb either!). They develop exceptional abilities to devise private mental stories to provide context for long complex word sequences.
C. Concepts: Logical Connections Of Facts
Unfortunately, flashcards are a poor way to master concepts. It is hard to understand some new concepts on the first encounter, and cards flashed into the attention do not help. Do you see where this is going? Understanding concepts requires a different strategy.
Here’s the good news. Some concepts give essential facts in ORGO contexts, if you nail that you have a little memory palace going on, chemistry style. Functional groups are much easier to remember for students who understand the concept that the same functional group in an infinite number of organic molecules has essentially the same reactivity because the importance of learning them becomes clear.
Similarly, connections between functional groups (eg aldehydes and ketones, or relationships between carboxylic acids, amides, nitriles, carboxylic acid anhydrides, and acid chlorides) provide a conceptual framework to functional group memorization.
Examples of concepts in ORGO include:
• hybridization
• valences of C, H, N, and S
• what curly arrows mean
• conformations of alkanes including Newman projections
OMG, OMG! These occur at the very beginning of sophomore organic! They do because they are used over and over again in the whole course.
Another piece of good news is learning concepts tends to be a lot more interesting than memorization. Oh sure, understanding concepts can be hard, but students get feel-good
moments when they conquer them.
If flashcards are near useless for understanding concepts, then how to do it? The box of tricks containing strategies to help contains:
• searching the web
• reading a textbook
• ask someone to explain things to you, and, very best,
• work out the concept for yourself or, more fun, with friends
In general, psychology says that if the concept is not too easy or too hard then students can grapple with the challenge, develop some learning flow, and eventually harvest that feel-good moment mentioned above (affiliate link below for a book Flow written by the person who popularized this concept and also to Deep Work which applies it in an academic setting). The challenge has to be Goldilocks perfect, though: not too cold and not too hot.
An example of combinations of memorization and conceptual understanding is remembering the mathematical equation relating pH values and hydrogen ion concentrations then applying a conceptual understanding to deduce how to relate this to Ka. When a concept is used and taken out of the context in which it is taught, that is a sign of mastery. It also is borderline with applications.
D. The Third Type Of ORGO Learning: Application
Application is using facts and/or concepts to arrive at an answer to a problem framed in a very specific context. Extrapolating from above, if we remember the mathematical equation relating pH values and hydrogen ion concentrations, then apply that concept to calculate the pH of a 1M solution of acetic acid in the water, that is an application.
Memorization and concepts probably only account for about 5 – 10% each of sophomore organic. When they are mixed up with the other 90 – 80%, everything should get easier still, after getting into the flow enough times.
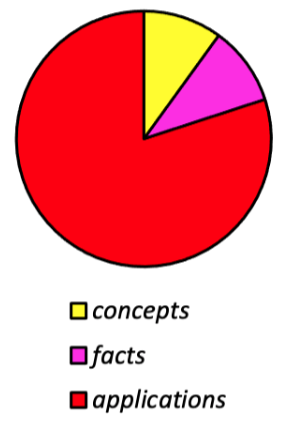
Fig 1. Types of organic chemistry. Different learning (and teaching) strategies are optimal for all.
We cannot use the same learning techniques to conquer facts, concepts, and applications efficiently.
techniques used to study facts, concepts and applications are different
Consider the following questions.
What is this functional group …..? Define pKa. What frequency is the carbonyl infrared stretch for acetone?
Functional group structures have to be learned and memorized. Defining pH requires memorization. Remembering the CO stretch for acetone too. All that is memorization.
However, what about …..
“Which of these functional groups is most reactive toward soft nucleophiles?”
“How does pH relate to pK an in water.”
“Why is the carbonyl IR stretch for ketones more than for most lactones”.
Answering those types of questions is a three-step process: (i) remembering some key facts; (ii) understanding key concepts; and, (iii) putting those together to solve problems that may seem unfamiliar at first. Congratulations! Some of you have not remembered the key facts, do not understand the concepts, and in that case, awesome! Those are the ones who have successfully screwed up because they will not be able to solve problems. For them, the temptation is to gravitate towards “feel-good” study strategies: the easiest ones to do quickly.
Many students simply study in the wrong way, not just for organic chemistry but many other subjects too, and reading the textbook over and again, highlighting chunks at a time, highlighting
it all! This is one of the most common ways. That will not well at all in organic chemistry, because so much of it is an application of memories and concepts.
Hands up if you have never seen a can-opener. Goooooood. I don’t see any hands going up.
Now, grab a piece of paper and draw a diagram to illustrate how one works. A lot of us don’t know. We see these situations frequently, they look so familiar we all think we know, but we don’t. That, in psychology, is The Illusion of Fluency.
In theory, you know it, in practice you don’t.
In life there are things we know; some we don’t know and know we don’t, and others that we don’t know and don’t know we don’t. No great insights there.
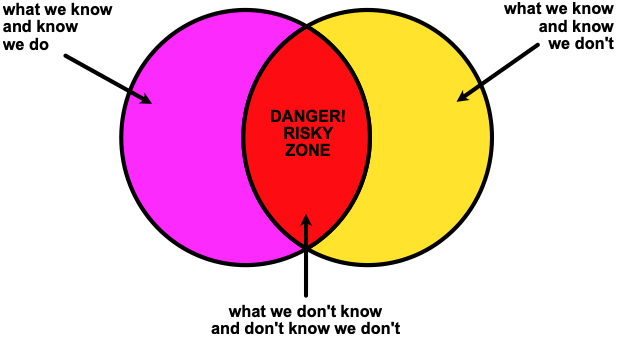
Fig 2. Beware of the risky zone.
Often we think we remember and understand the basics well enough to apply them in any situation. Perceptions of ourselves are often wrong! We cannot know what we do not know, hence can be poor judges of our own mastery of a subject. Always there are subsets of the information that we think we know and don’t; these are risky areas. Applications in organic chemistry often fall into that blind spot which highlights things we do not know and don’t know we don’t.
An Inconvenient Truth.
The first five to ten lectures of sophomore organic are loaded with facts to remember and concepts to understand. Students who do not remember or understand get punished repeatedly because they keep coming up in the rest of the course to bite the unsuspecting in the
proverbial ….. behind. For them, points hemorrhage in every quiz and test after that.; they have screwed up, royally. Why should that be so?
it is impossible for someone to apply facts which they cannot recall,
and concepts they do not understand
Sophomore Organic Chemistry By Inquisition 1 and 2 are workbooks not a textbook. In fact there is a little picture that says so (Fig 3):

Fig 3. Sophomore Organic Chemistry By Inquisition, book 1, has an attitude.
It is all questions and can be used for flipped classes. The answers are on the web. For learning facts, the questions are just like flashcards, except that there is a little energy barrier making it harder to jump to the answer too quickly. It tests understanding of concepts. Most though, it has lots of and lots of application questions to practice, many requiring spaced repetition of requisite facts and fact/concept combinations. Styles of applications can easily be interleaved using this book.
The contents of Sophomore Organic Chemistry By Inquisition 1 cover those critical opening lectures in ORGO are given in Fig 4.
1 Hybridization: The Shape Of Things To Come 15
A Introduction 15
B Electron Counting 16In Atoms 16
In Molecules, and Valency 17
C Mixing Atomic Orbitals To Maximize Overlap In Molecules 19
Combining s- and p-Orbitals 19
Geometric Shapes 19
Shapes Of Molecules Based On Geometric Shapes 20
D Multiple Bonds 23
2 Saturated Acyclic Hydrocarbons 28
A Introduction 28
B Conformations Of Acyclic Hydrocarbons 29
Ethane 29
Propane 31
Butane 33
Art In Organic Chemistry 35
Three Dimensional Diagrams Of Organic Molecules 39
Alkyl Fragments 40
C Conclusion 43
3 Fragments And Functional Groups 44
A Introduction 44
B Fragments 45
C Expanded Forms Of Functional Groups 48
D Abbreviated Forms Of Functional Groups 50
4 Conformations Of Cyclic Hydrocarbons 55
A Introduction 55
B Angle Strain 56
C Torsional Strain 56
D Cyclohexanes 57
Unsubstituted Cyclohexane 57
Monosubstituted Cyclohexanes 62
Disubstituted Cyclohexanes 64
Cyclohexenes 66
E Other Rings 67
5 Curly Arrows And Electron Flow 68
A Introduction 68
B Electron Flow 69
Affecting Only One Bond 69
Affecting Two Bonds 72
Affecting Four Bonds 73
Representations Of Charged Hydrocarbon Scaffolds 74
C Heteroatoms, Lone Pairs, And Moving Electrons 76
6 Acids And Bases 80
A Introduction 80
B Log Scales To Measure Proton Dissociation From Organic Molecules 81
Equilibria That Generate Protons 81
Simplifying The Scale: pKa 84
C Acid-Base Equilibria 85
D Predicting Relative pK a Values 89
E Predicting Sites Of Protonation 90
F Lewis Acids And Bases
Fig 4. Despite his strong attitude, Sophomore Organic Chemistry 1 has a lot of ways to test ORGO understanding.
E. Conclusion
Students eager to screw up their chance to do well in ORGO should:
(i) Never use flashcard software like ANKI to help remember facts. Never, ever hang out with a bunch of diligent friends, show each card, pause while everyone writes down the answer, then have someone reveal the back of the card; that is particularly bad because people tend to share what they thought and understand from each other how they screwed up.
(ii) Never test if you can work through a concept for the case it was shown in a lecture, then reason how it would be applied to unfamiliar situations. This is so obviously good for understanding that I will not explain why.
(iii) Skip the first 5 – 10 lectures of ORGO and sleep in.
(iii) Don’t take opportunities to apply facts and concepts to many different examples of varying kinds. If you do, check the answers immediately without thinking about how to solve the problem. At all costs, avoid using cases where you got it wrong to check to understand of
the key facts and concepts.
(iv) Never buy a copy of my workbooks, Sophomore Organic Chemistry By Inquisition 1 and 2, (ah-hem, from byinquisition.org for $35 per book, or in suck-it-and-see sampler parts on Apple Books for a lightweight $9.99 each enabling problem solving on an iPad, or I guess via Amazon {in which case Jeff takes a big cut}). Don’t do this because it allows spaced repetitions of quizzes about key facts to remember, gives frameworks for reproducing concepts, and lots and lots of application examples with the answers inconveniently accessible on the web to discourage looking at them straight away.
Nearly almost every sophomore ORGO class begins with lots of facts and concepts, then moves into applications for most of the rest of the course. Students do not like remembering seemingly pointless facts or grappling with difficult concepts that may be only useful once.
ORGO is hard for students who bypass essential memorization and conceptual understanding, particularly at the beginning of the course semester one, and thereafter do not recognize that a different style of learning, solving different problems to achieve mastery, is required for 80% of the course.
Ciao